Brachy, which in Greek means "short", is used in the context of therapy at short distances. G. Forssell coined the term for the first time in 1931. Since then, various other terms have been used to define this treatment mode using radionuclides, such as, plesiotherapy, endotherapy, curietherapy, endocurietherapy, etc. Brachytherapy is the internal radiation treatment achieved by the placement of sealed radioactive sources into or in the proximity of the tumor, allowing the patient to receive radiation therapy from the inside out. This mode of treatment started immediately after the discovery of radium by the Curies in 1898. Dr. Danlos conducted the first recorded treatment in 1901 by the insertion of a glass tube containing radium sulfate into a tumor. Early pioneers resorted to the method of inserting bulky radium tubes within the tumor for a certain period of time and withdrawing them. However, it was with the advent of artificial radioactivity that brachytherapy became so popular. Brachytherapy allows the delivery of a higher biologically effective dose of radiation to the tumor with less normal tissue damage in a shorter time than is possible with external radiation. By placing the radiation source or sources inside or immediately adjacent to the volume of the tissue to be treated, the dose to the other tissues can be minimized because the radiation does not have to pass through any other tissue before arriving at the treatment volume. inside the treatment volume, the dose is non-uniform and the dose gradients are often high. There are certain systems like the Manchester technique, which are designed for dose uniformity. Brachytherapy is limited to accessible sites such as near the surface of the body or near the natural body cavities having small volumes. The dose rates in brachytherapy are usually low compared to external beam therapy. Brachytherapy is used as a primary treatment or as a supplementation for external megavoltage radiation therapy. The advantage of brachytherapy is that it is less invasive than surgery and often has fewer side effects when compared with other procedures. Also the recovery time is very short and the patient's quality of life is not impacted much. External radiation on the other hand, though less traumatic than surgery, is extremely time consuming and requires 4-8 weeks of five-times-a-week treatments. Nevertheless, in external beam therapy, a high degree of dose uniformity can usually be achieved in the treatment volume.
Types of Brachytherapy
The success of brachytherapy depends on the method used for delivering the effective dose of radiation and selection of the appropriate radionuclide for treatment. The technique of brachytherapy may be divided into three distinct applications: surface moulds, interstitial implants, and intracavitary insertions. Surface Mould Applications Surface moulds or plaques are usually employed in cases of superficial lesions, wherein the radioactive sources are arranged on the external surface of the patient, displaced slightly from the lesion. The source is then mounted on a wax or plastic piece to fill in the space between the source and the lesion. The advantage of this treatment is the rapid fall off of dose thereby saving the sensitive normal tissue below, if present. The roost common use is in the treatment of ocular tumors with the help of beta and photon emitting radionuclides. Owing to the advancement in the treatments using electron beams and superficial x-rays, this mode of therapy is being slowly replaced. Interstitial Implants The radioactive sources used in interstitial brachytherapy are surgically inserted directly into the diseased tissue or into the tissue adjacent to the lesion but not in a body cavity. The sources usually have small diameter to allow penetration into the tissue. The commonly used radionuclides in interstitial brachytherapy are Ir-192 and 1-125. They are used for treating cancers of accessible sites like the prostate, intra-oral cancers and superficial tumors. The common dose rates prescribed range from 7-20 Gy/day over a span of 2 - 10 days. Intracavitary Insertions In intracavitary therapy, radioactive sources such as Co-60 or Ir-192 are introduced into the body cavity in a form-fitting applicator to irradiate the cavity walls and any lesion therein. The common cancers treated are those of the cervix, uterus, vagina, rectum, nasopharynx and esophagus. Once the applicator has been fixed in position, the radioactive sources can De loaded into it manually or remotely. High dose rate remote after loading brachytherapy units allow treatment in a few minutes using a high activity (typically 10 Ci) radioactive source that travels through the catheters to the tumor. Since the source is moved from a shielded safe to the desired position inside the patient by remote control, the dose to the staff is insignificant.Characteristics of Brachytherapy Radionuclides
The suitability of a radionuclide for brachytherapy is determined by its half-life and by the type, energy, and branching ratios of its emissions. Radioisotopes emitting beta and gamma rays are usually employed in brachytherapy as they have more penetrating power than alpha rays. Although beta rays do not penetrate more than 3-4 mm, they are useful for the treatment of superficial lesions like those of the skin and eye. Gamma emitters are usually preferred in brachytherapy because of their penetrating power. Radium was used formerly, but has been replaced because of the hazard caused by radon gas leakage. Ir-192 and 1-125 are used in the majority of interstitial brachytherapy treatments. The radionuclides are sealed in suitable encapsulation. This covering acts as a filter to absorb any unwanted low energy radiation emitted along with the main energy, as in the case of gamma radiation being accompanied by low energy betas. The covering is usually made of titanium or an alloy of platinum and iridium. The high atomic number and density of these materials ensures the absorption of all the unwanted alpha and beta emissions Even with the encapsulation, the sources must have a small size to ease the process of application. The problem here is that the source activity is directly proportional to the number of radioactive atoms present and inversely proportional to the half-life. For a given activity requirement, a longer half-life requires a greater number of atoms and therefore a larger source. Hence, as a compromise between the constraints, the source for a particular application is selected depending on the type of implant - temporary or permanent. Temporary implants are used for tumors that are readily accessible from opposite sides as in the neck, breast, and skin. The temporary implants mostly use Ir-192 and Cs-137 and are completed within a few hours or days. Permanent implants use individual single seeds or seeds loaded in magazines, which can be inserted into the tumor In permanent implants, the radioactive source remains in the patient indefinitely and hence sources having short half-lives like I-125 are preferred. This form of therapy aids in controlling fast growing tumors. Permanent implants are utilized mainly for the treatment of the tumors of the brain, prostate, lung and some gynecological sites.
Sr-90 Plesiotherapy |
| Follow this link for a list of articles describing plesiotherapy in veterinary medicine. |
 |
|
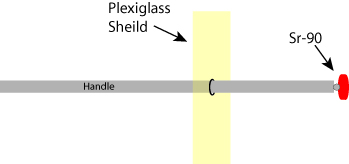
Figure 1: Schematic of a Sr-90 applicator. The sealed strontium-90 radioactive source is mounted on a rod that has a flexible hinged end, allowing the active head to be rotated 1800. The strontium source is held by the rod, and a plexiglass shield protects the operator from radioactive Beta particles emitted by the strontium-90 source.
|
 |
|
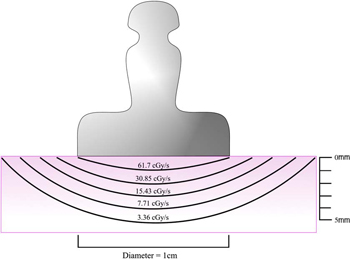
Figure 2: Dose distribution curves for a Sr-90 applicator. Present day applicators use Y-90 (T1/2 = 64 hours) in secular equilibrium with its parent Sr-90 (TI/2 = 28 years). The front surface of the applicator absorbs most of the low energy beta particles from Sr-90 (0.54 MeV), but permits the high energy beta from Y-90 (2.27 MeV) to enter the eye. The dose rate at the center of an applicator surface may be as high as 100 cGy/s. The dose rate near the applicator decreases to about 50% at a depth of 1 mm in tissue and becomes 5% of the surface dose rate at a depth of 4 mm.
|
 |
|
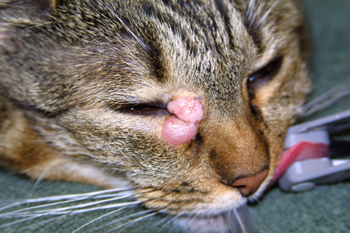
Figure 3: Photograph of a biopsy confirmed cutaneous mast cell tumor (MCT) involving the medial canthus of the right eye.
|
 |
|
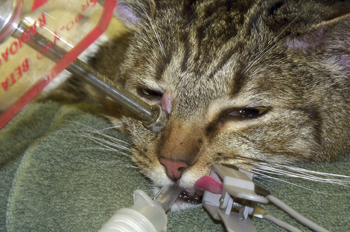
Figure 4: Photograph of the cat in Figure 3 above undergoing treatment for a cutaneous MCT. The strontium-90 ophthalmic applicator is applied to the surface of the tumor on the medial canthus.
|
 |
|
Figure 5: Six (6) week post treatment follow up picture of the cat in figures 3 & 4 above showing the resolution of the mass. Note the hyperpigmentation and hair loss in the irradiated area.
|
 |
|
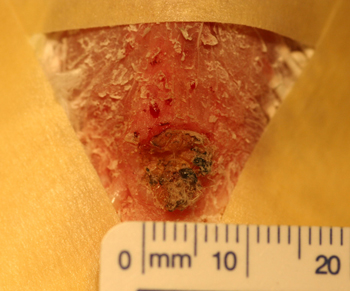
Figure 6: Post biopsy picture of the surgical site after removal of a squamous cell carcinoma involving the uropygeal gland of a 21 year old lutino cockatiel.
|
 |
|
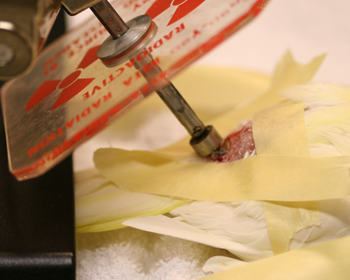
Figure 7: Sr-90 therapy of a squamous cell carcinoma involving the uropygeal gland of a 21 year old lutino cockatiel.
|
 |
|
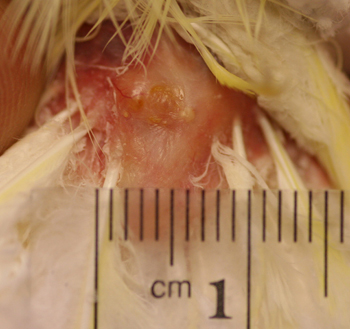
Figure 8: Six month post therapy picture of the uropygeal gland of a 21 year old lutino cockatiel. Note the normal appearance of the gland and surrounding skin.
|
 |
|
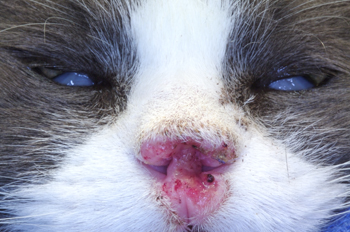
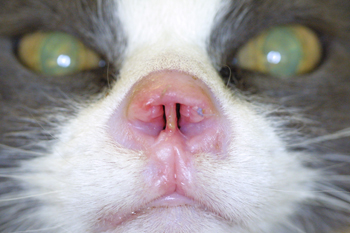
Figure 9: Pretreatment picture of an aggressive squamous cell carcinoma of the nasal planum of a cat. Notice the ulcerated surface of the skin and erosive nature of the disease. In addition there is swelling of the adjacent surrounding haired skin in reaction to the neoplasia.
|
 |
|
Figure 10: Six week post therapy picture of the nose of a cat with squamous cell carcinoma of the nasal planum. Notice the marked resolution of the ulcerated mass which has been replaced by newly epithelialized skin devoid of hair.
|

